- Stearate series
- · calcium stearate
- · Zinc stearate
- · Magnesium stearate
- · Sodium stearate
- · Potassium stearate
- · Lithium stearate
- · Aluminium stearate
- · Stearic acid
- MCC series
- · MCC CP2010
- · MCC PH101
- · MCC PH102
- · MCC PH105
- · MCC PH103
- · MCC PH112
- · MCC PH113
- · MCC PH200
- · MCC PH301
- · MCC PH302
- Colloid MCC series
- · Colloid MCC
- Acetylacetone salt series
- · Calcium(III) Acetylacetonate
- · Zinc(II) Acetylacetonate
- Oleate series
- · Sodium oleate
- · Potassium oleate
- Laurate series
- · Sodium laurate
- · Zinc laurate
- Myristate series
- · Magnesium myristate
- · Sodium myristate
- Water-based emulsion
- · Water-based zinc stearate em
Detail
Medicinal materials
Molecular formula: CH3(CH2)16COONa
CAS NO. : 822-16-2
Molecular structure:
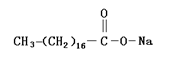
Main uses
In medical industry, it is used as lubricating agent, and adhesion agent.
Quality standard
|
Testing item |
Testing standard |
|
|
Specification |
USP35-NF30 |
BP2013 |
|
appearance |
white or light yellow fine powder |
white or light yellow fine powder |
|
identification |
meet the specification |
meet the specification |
|
solubility |
/ |
meet the specification |
|
PH value |
/ |
meet the specification |
|
chloride, % |
/ |
≤0.2 |
|
sulfate, % |
/ |
≤0.3 |
|
acid value |
196-211 |
/ |
|
sodium content, % |
/ |
7.4-8.5 |
|
iodine value, % |
≤4.0 |
/ |
|
acid value% |
0.28-1.2 |
/ |
|
loss on drying, % |
≤5.0 |
≤5.0 |
|
insoluble in alcohol |
meet the specification |
/ |
|
nickel, ppm |
/ |
≤5 |
|
stearic acid content % |
≥40.0 |
≥40.0 |
|
stearic acid&palmitic acid content % |
≥90.0 |
≥90.0 |
|
bacteria, cfu/g |
/ |
≤1000 |
|
mould, cfu/g |
/ |
≤100 |
|
pathogenic bacterium |
/ |
not detectable |
Packaging
woven bag lined high pressure polyethylene film; 25kg/bag or follow customer's packing instructions
| 【Last】 【Next】 【Return】 |


